2021 Nobel Prize in Physiology on the Role of TRP Family of Ion Channels
BCDD team in collaboration with the Cardiovascular Research Laboratory at Sourasky Medical Center, study the TRPV2 channel as part of the fascinating TRP quest
Dr. David Julius (UCSF) and Dr. Ardem Patapoutian (Scripps Institute) recently received the 2021 Nobel Prize in physiology for their discoveries of the fascinating roles that the Transient Receptor Potential (TRP) family of ion channels play in temperature and touch sensation. In the late 1990s, David Julius wished to identify the cellular target of capsaicin, the pungent ingredient of chili peppers, as he believed this could provide fundamental insights into mechanisms of pain. He used a cDNA library from sensory neurons in a functional screen to look for a gene that could confer capsaicin sensitivity to cells that were normally unresponsive. The screen identified the cDNA encoding the TRP vanilloid 1 (TRPV1) cation channel. TRPV1 was soon shown to be activated by elevated temperatures (520C) perceived as being painful. Dr. Julius showed that an ectopic expression of TRPV1 in cells followed by induction with capsaicin evoked electrophysiological properties that resemble those of channels found in native sensory neurons.
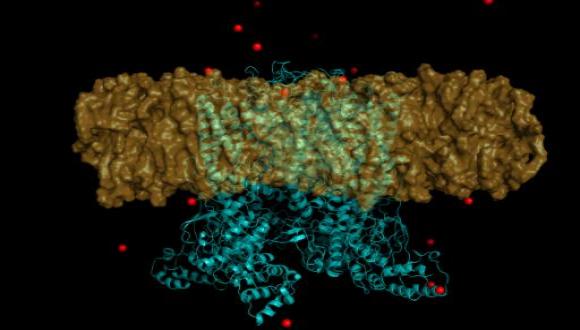
Several additional TRP-receptor channels were subsequently identified and shown to transduce thermal information in the somatosensory system. Among which is its close family member TRPV2 that is activated upon 42C° and shares 50% homology to TRPV1. In parallel, Patapoutian identified a cell line that gave off a measurable electric signal when individual cells were poked with a micropipette. It was assumed that the receptor activated by mechanical force is an ion channel and in a next step the candidate genes encoding possible receptors were identified. These genes were inactivated one by one to discover the genes responsible for mechano-sensitivity in the studied cells- named Piezo1/ Piezo2 (after the Greek word for pressure). Later on, both Julius and Patapoutian identified TRP8- which is activated by cold temperatures.
Related Studies at the Tel-Aviv University
In 2012, The Cardiovascular Research lab at the Tel-Aviv Sourasky Medical Center, headed by Prof. Gad Keren and Dr. Michal Entin-Meer, found that, among the recognized cardiac channels, the TRPV2 gene is significantly and exclusively overexpressed at the peri-infarct zone in murine and rat models for acute myocardial infarction (AMI). Through a series of in vivo experiments followed by echocardiography measurements and immunohistochemical analyses, the researchers showed that TRPV2 is mainly expressed on mature macrophages accumulating around the infarct zone and that the intact form of TRPV2 mediates an unfavorable recovery from the ischemic event due to stormy immune reactions.
In view of their findings, we initiated a collaborative study between the Medicinal Chemistry (Dr. Elvira Haimov), High Throughput Screening (Dr. Eddy Pichinuk) and Computer-Aided Drug Design (Dr. Luba Ischakov) units of the BCDD and the cardiology lab. We are currently developing novel small organic molecules that potently and selectively inhibit TRPV2 and thus may ameliorate progression of inflammatory diseases including: AMI, inflammatory bowel disease, inflammatory skin disease, etc.
This collaborative research is funded by the Kamin innovation award (2020-2023). We believe that the novel TRPV2 blocking molecules that are going to be presented soon will be highly efficacious in attenuating inflammatory injuries and the pain associated with these illnesses. These findings may shed light on the crucial roles of the TRPV channels under physiology and pathology.